-
Chromatographic Resolution, Solution and Crystal Phase Conformations, and Absolute Configuration of tert-Butyl(dimethylamino)phenylphosphine-Borane Complex
J.-V. Naubron, L. Giordano, F. Fotiadu, T. Bürgi, N. Vanthuyne, C. Roussel and G. Buono
Journal of Organic Chemistry, 71 (15) (2006), p5586-5593


DOI:10.1021/jo0605647 | unige:14781 | Abstract | Article HTML | Article PDF
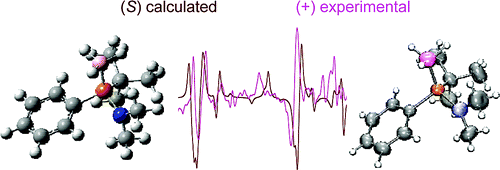
The enantiomers of tert-butyl(dimethylamino)phenylphosphine−borane complex 2 have been separated by HPLC using cellulose tris-p-methylbenzoate as chiral stationary phase. The borane protection could be removed without racemization and the P-configuration of the free aminophosphine 1 has shown to be stable in solution. Infrared (IR) and vibrational circular dichroism (VCD) spectra have been measured in CD2Cl2 solution for both enantiomers. B3LYP/6-31+G(d) DFT calculations allowed a prediction that complex (S)-2 exists as three conformers in equilibrium and computed population-weighted IR and VCD spectra. Predicted and experimental IR and VCD spectra compared very well and indicate that enantiomer (+)-2 has the S absolute configuration. This assignment has been confirmed by an X-ray diffraction study on a single crystal of (+)-2. The crystal structure of enantiomerically pure 2 appears to be very close to the most stable computed conformer which proved to be predominant in solution.